Simply because these cookies are strictly essential to provide the website, refusing them will have impression how our web-site features.
Nonetheless, the necessity for any technical file may well range based on the EU classification of medical devices.
In the situation of One Use Devices (SUDs), specially in the chance management documentation, suppliers shall display and substantiate why the device is produced as a result – that is, it really should Evidently be stipulated why the device cannot be reprocessed3.
As it's unachievable to provide an individual illustration of a technical document, this information gives some illustrations for the most common technical documents.
Complexity: It’s simpler to assess compliance based on A fast DoC and take a look at report overview, when compared to building that same evaluation on the technical documentation.
There are lots of different technical conditions, paperwork and acronyms that it might look like a little bit of an alphabet soup. We’d like to address a few paperwork that result in a great deal of confusion and tearing outside of hair; the technical file, 510(k) submission and the design history file.
Take a look at the most up-to-date enhancements and innovations in prostate imaging that are reworking diagnosis and therapy.
“If you've a Model just one of the solution and also you're now launching Model two you could simply ‘archive’ the whole Variation of that technical file. So it goes from being the medical device file to currently being the Design Record File Edition in practically one particular simple motion.”
The assessment system for a medical device technical file Medical Device Technical File entails a comprehensive evaluation of the documentation by a Notified Body, which can be a designated Business liable for verifying the compliance of medical devices with regulatory needs.
In some cases it feels like you need to know a completely new language to work while in the medical device industry.
Importantly, non-EU makers ought to maintain the file with a certified agent within the EU for talk to Anytime requested – as a result, retain a managed version in the file generally readily available[three].
The design and producing information and facts doc incorporates facts with regard to the device’s style history, producing processes, and supplies used. The security and effectiveness specifications doc outlines the device’s compliance with appropriate specifications and regulations, as well as merchandise verification and validation info doc consists of exam studies and scientific investigation info.
You might also elect to use external consultants to assist you to should you don’t have The interior abilities available. It may be invaluable to get an individual are available who now has expertise in your certain sort of task.
In case a medical device malfunctions or leads to hurt, the technical documentation also will help locate and repair the issues, safeguarding public health and fitness and holding the company’s track record intact.
 Shaun Weiss Then & Now!
Shaun Weiss Then & Now! Kelly Le Brock Then & Now!
Kelly Le Brock Then & Now!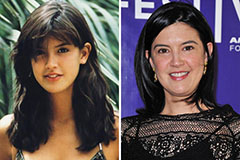 Phoebe Cates Then & Now!
Phoebe Cates Then & Now! Jeri Ryan Then & Now!
Jeri Ryan Then & Now! Nicki Minaj Then & Now!
Nicki Minaj Then & Now!